Iron manganese copper lead and zinc are commonly found in acidic water. 22 Food Food is the principal dietary source of intake of both calcium and magnesium.

Tolerance Limits For A Few Metal Ions In Drinking Water 40 Download Table
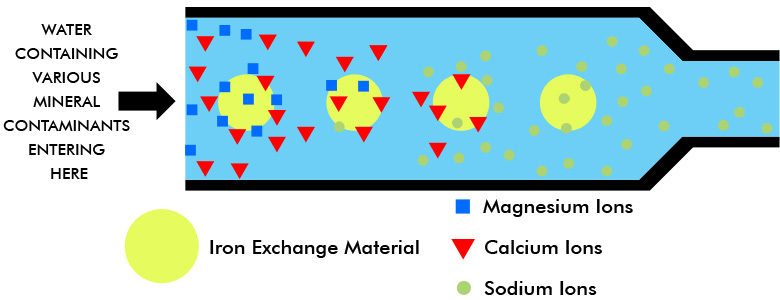
The Ion Exchange Principle Apec Water
Simplification Of Metal Ion Analysis In Fresh Water Samples By Atomic Absorption Spectroscopy For Laboratory Students
Water fluoridation is the controlled adjustment of fluoride to a public water supply solely to reduce tooth decayFluoridated water contains fluoride at a level that is effective for preventing cavities.

Ions in drinking water. The pH Misconception. Simply connect and download the app to your phone. Drinking water that is slightly alkaline is great and all but most of the benefits lie in the ionized.
Fluoridated water operates on tooth surfaces. Ions are atoms or molecules that have a net positive or net negative charge. A water softener will provide you with clean clear.
The pH of water can indirectly affect your health. It is recommended that the allowable limit should be applied because water exceeding those values mentioned under Acceptable is not appropriate. As a result the synthesised nanoparticles can be used as sensors to detect heavy metal ions in water.
The process uses aluminum sulfate a coagulant normally used for water treatment to flocculate fluoride ions present in the drinking water. HARDNESS IN DRINKING-WATER 2 Estimated daily intakes of magnesium from water of about 23 mg and 521 mg in soft-water and hard-water areas respectively have been reported based on adults drinking 2 litres of water per day Neri et al 1985. Several new techniques for softening water without introducing sodium ions are beginning to appear on the market.
Thus the builder must exhibit high capacity for selective removal of these ions from water. The reports are often sent out with water bills but they may be sent separately. A PDF version of this document with information conveyed visually in table format is available please note that the PDF is not accessible for screen readers.
Because water softened by this type of ion exchange contains many sodium ions people with limited sodium intakes should avoid drinking water that has been softened this way. Using Bluetooth technology IONs are available with the ability to dispense water using your smartphone. The experiments also reported the selective luminescence quenching by heavy metal ions.
Page 4 Interpreting Drinking Water Quality Results Saturation Index The saturation index is a measure of waters ability to corrode or form scale. Sodium ions are supplied from dissolved sodium chloride salt also called brine. Unlike conventional drinking water pH 88 alkaline water instantly denatures pepsin rendering it permanently inactive.
In the mouth it creates low levels of fluoride in saliva which reduces the rate at which tooth. 91 US fl oz per day for human females older than 18 which. This HTML page contains the same information as that found within the PDF.
ION drinking water appliance a unique new bottle-free water cooler that makes all other water coolers obsolete. Standard water softeners are cation exchange devices. However in areas where the drinking water is contaminated to a level of 50 ppmfive times the MCL for nitrate nitrogendrinking water may supply as much as half of a persons total daily intake.
This can occur naturally or by adding fluoride. More communities will consider desalination of brackish and salt water to produce drinking water. The dissolved inorganic salts of calcium and magnesium impart hardness to water.
A water softener is an ion-exchange system that removes calcium and magnesium ions from hard water. Because water softened by this type of ion exchange contains many sodium ions people with limited sodium intakes should avoid drinking water that has been softened this way. Together the hypochlorous acid and the hypochlorite ions are referred to as free chlorine.
It is calculated using values from pH alkalinity total hardness and conductivity tests. And other developed countries. This discovery has a potential to develop efficient sensors to monitor heavy metal ions in drinking water.
A water softener typically uses sodium or potassium ions to replace calcium and magnesium ions the ions that create hardness Distillation Systems Distillation is a process in which impure water is boiled and the steam is collected and condensed in a separate container leaving many of the solid contaminants behind. High levels of lead in drinking water is a primary concern of pH. Thus the consumption of alkaline water may have therapeutic benefits for patients with reflux disease.
As water flows through the unit the resin releases its sodium ions and readily trades them for the calcium and magnesium. Water with acidic pH levels can corrode plumbing and leach metal. Several new techniques for softening water without introducing sodium ions are beginning to appear on the market.
Estimated total exposure and relative contribution of drinking-water If a daily water consumption of 2 litres and an average chloride level in drinking-water of 10 mglitre are assumed the average daily intake of chloride from drinking-water would be approximately 20 mg per person 4 but a figure of approximately 100 mgday has also been. A negative value indicates that water is likely to be corrosive while a positive value indicates a tendency. A Guide to Drinking Water Treatment Technologies for Household Use pdf icon PDF 126 MB.
These drinking water quality standards describe the allowable limit and permissible limit in the absence of an alternate source. Four common ionic molecules in saline water are sodium chloride calcium and carbonate. The best way to learn about your local drinking water quality is to read the annual drinking water quality reportconsumer confidence report that water suppliers now send out by July 1 of each year.
In addition it has good acid-buffering capacity. In drinking water. Water softeners usually use sodium Na as the exchange ion.
A pH between 65 and 85 will see both hypochlorous acid and hypochlorite ions present in the water. Drinking water quality standards gives the important quality parameters set for drinking water. This document is designed as a guide for.
When people begin saying that the higher the pH of drinking water the better it only fuels the naysayers. The reports tell where drinking water comes from what contaminants are in it and at what levels. A softener filters calcium and magnesium laden water through a resin coated with sodium ions.
A lot of times when people say they are drinking alkaline water for their health they forget to mention the ionized part of alkaline ionized water. Drinking water generally accounts for 5 to 10 percent of the nitrates that people consume. Before cities began routinely treating drinking water with chlorine starting with Chicago and Jersey City in 1908 cholera typhoid fever dysentery and hepatitis A killed thousands of US.
Cations refer to positively charged ions dissolved in the water. The amount of drinking water required per day is variable. Hypchlorous acid is the more effective disinfectant.
Cation exchange involves the replacement of the hardness ions with a nonhardness ion. 130 US fl oz per day for human males older than 18 and 27 litres 95 imp fl oz. The system is made up of two 20 liter plastic buckets each having a small brass tap fixed about 5 cm above the bottom part to enable trapping of the sludge just beneath the draw-off point.
It depends on physical activity age health and environmental conditionsIn the United States the Adequate Intake for total water based on median intakes is 37 litres 130 imp fl oz. Drinking water chlorination and filtration have helped to virtually eliminate these diseases in the US. For nitrate removal the resin exchanges chloride ions for nitrate and sulfate ions in the water.
For the washing process to proceed effectively it is important to remove the hardness ions in the water and replace them with sodium ions rendering softness to the water.
1

Does The Ph Level Of Your Drinking Water Really Matter

Is Alkaline Water Really Better For You The Washington Post

Pocari Sweat Ion Water Otsuka Pharmaceutical Co Ltd

Understanding The Benefits And Risks Of Deionized Di Water

Pocari Sweat Ion Water Otsuka Pharmaceutical Co Ltd

Measurement Of Calcium In Drinking Water Horiba

Simplification Of Metal Ion Analysis In Fresh Water Samples By Atomic Absorption Spectroscopy For Laboratory Students Semantic Scholar
